Melting And Boiling Point Npentane Nhexane Stock Illustration - Download Image Now - Acid, Alcohol - Drink, Atom - iStock
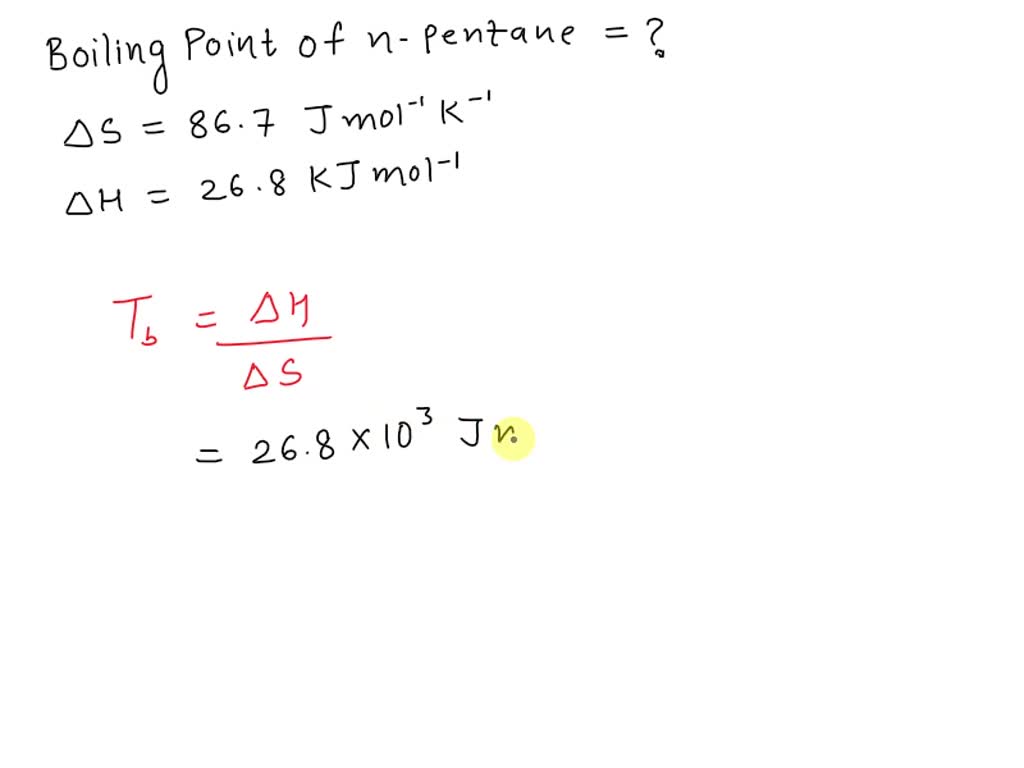
SOLVED: Calculate the boiling point (BP) of n-pentane, given that the = Points) entropy 14)(6= BPis86.7 Jlmol x *K and the change in enthalpy is 26.8 kJlmole change at the
Rank these compounds by boiling point from highest to lowest boiling point: pentane, neopentane, hexane - Home Work Help - Learn CBSE Forum
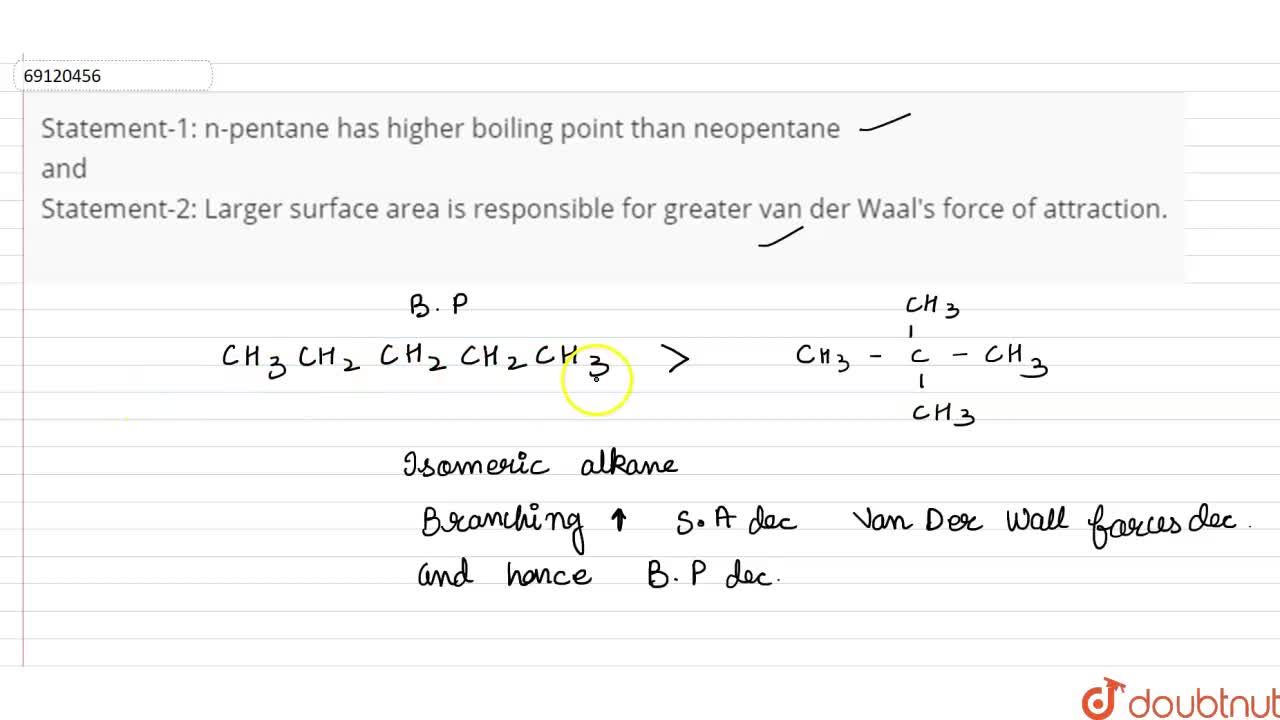
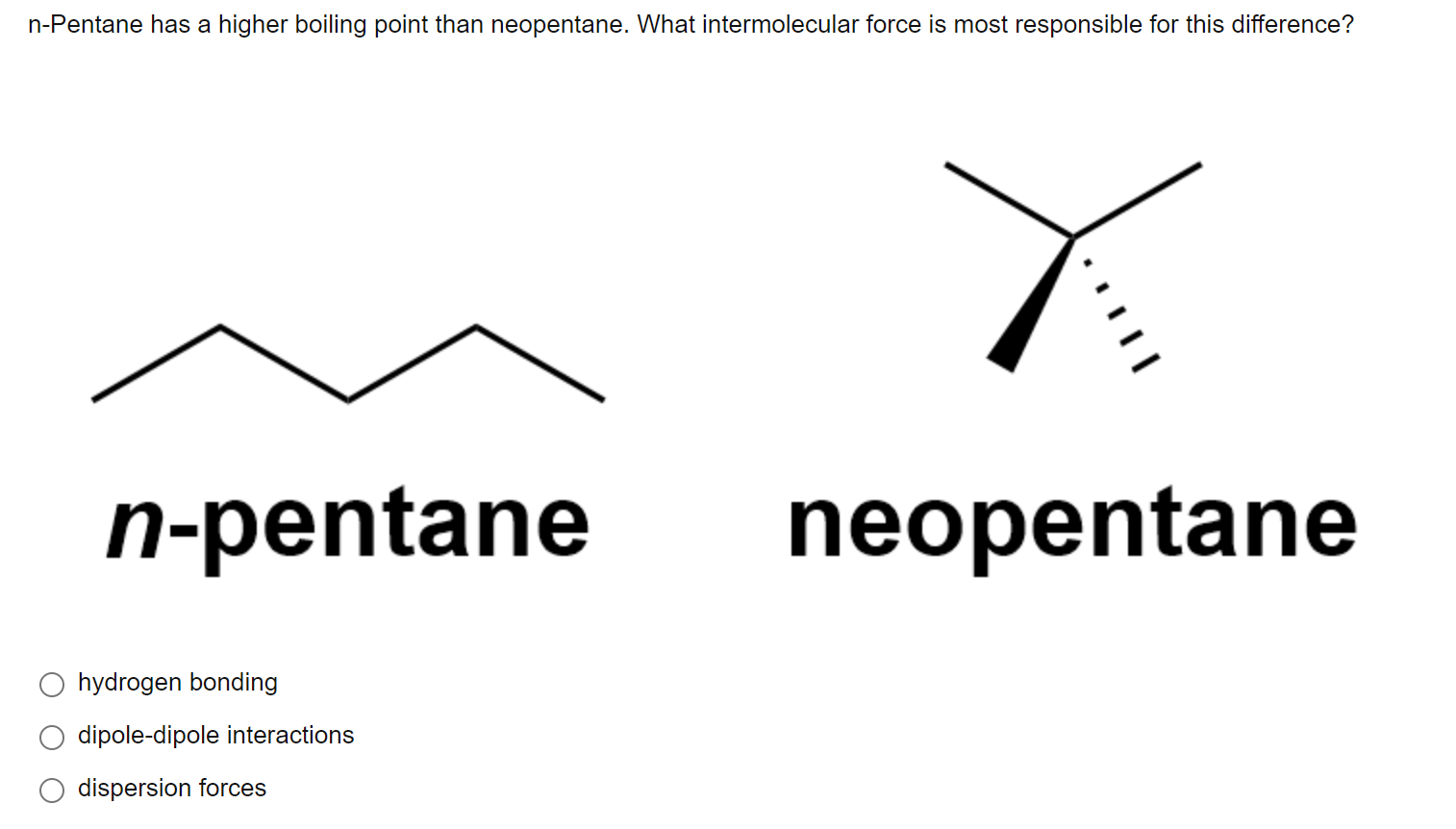
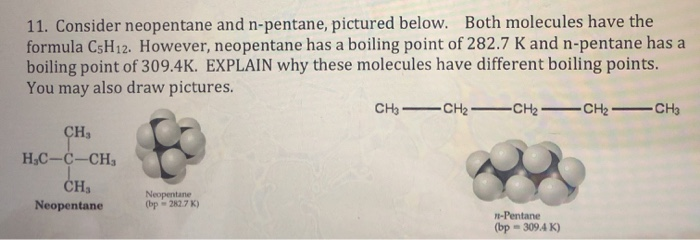
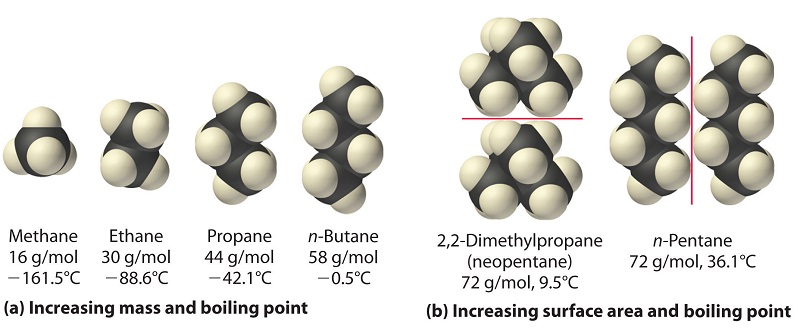
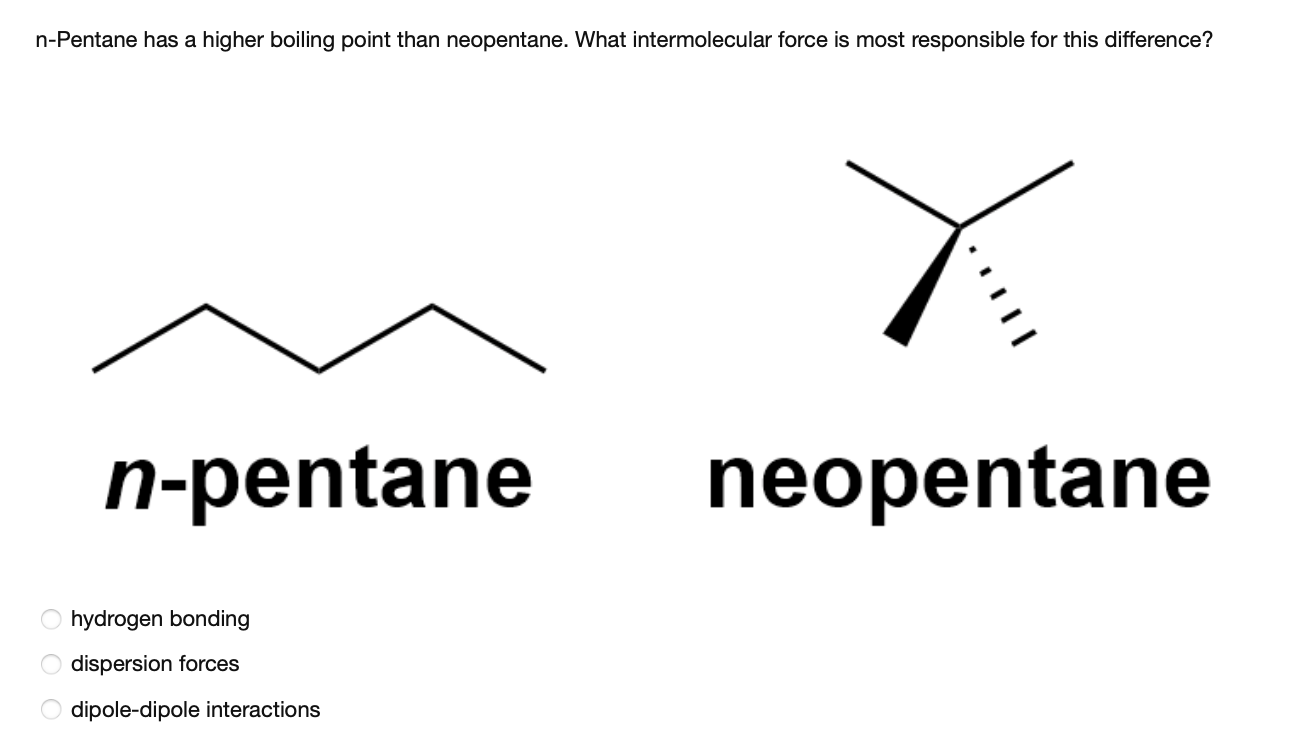
Statement-1: n-pentane has higher boiling point than neopentane and Statement-2: Larger surface area is responsible for greater van der Waal's force of attraction.
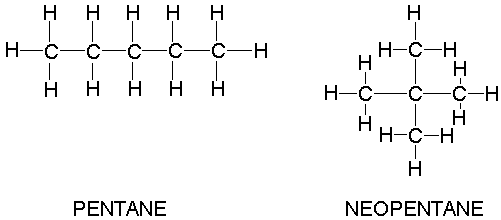
Why when the shape of molecules become more compact it's boiling point decrease while when intermolecular force become strong boiling point increase? | Socratic

Rank these compounds from highest to lowest boiling point. a. pentane b. neopentane c. isopentane | Homework.Study.com

why neopentane has higher melting point than n pentane - Chemistry - Chemical Bonding and Molecular Structure - 13416933 | Meritnation.com

organic chemistry - Why does neopentane have a higher melting point than n- pentane? - Chemistry Stack Exchange

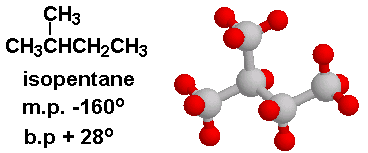
Explain why n-pentane has higher boiling point than neo pentane but melting point of neo-pentane is more than n-pentane

Arrange the following in decreasing order of their boiling points:(1) n - butane (2) 2 - methyl butane(3) n - pentane (4) 2,2 - dimethyl propane

Arrange the following compounds in the descending order of their boiling pointsa) n - pentaneb) isopentanec) neopentane